| Chazin Home | Ca-binding Protein DB | Vanderbilt Home |  |
|
| Research Description | Publications | Wisdom | Search | ||
| How to contribute | About this page |
Transform BL21 (DE3) pLysS cells with Calmodulin plasmid (PiCBWR, supplied by E.Thulin). Follow normal procedure for competent cells except: all incubations after the heat shock step should be at 30°C unless otherwise noted.
Plate transformation reaction on LB agar with 34mg/mL chloramphenicol and 100mg/mL ampicillin. Grow overnight at 30°C, or until colonies form.
Inoculate 20 mL of LB broth (with chloramphenicol and ampicillin as above), grow with shaking overnight at 30°C.
Inoculate 100 mL of LB/chloramphenicol/ampicillin broth with 4 mL of overnight culture. Measure absorbance of starting culture at 600nm. Grow/shake at 30°C until absorbance at 600nm doubles.
Inoculate 900 mL of LB/chloramphenicol/ampicillin broth with entire 100mL culture. Add IPTG to final concentration of 0.5mM. Grow/shake at 37°C until growth stops, about 4 hours.
Harvest cells by centrifugation, freeze cell pellet at -80°C until ready to perform lysis procedure.
Cell LysisResuspend cell pellet in about 10 mL Lysis Buffer (it will be a thick, gooey mess). Incubate suspension on ice for 30 minutes with frequent shaking.
Add about 60 mL Enzyme Buffer, incubate on ice with frequent stirring for 10 minutes.
Add 100 µg/mL lysozyme, incubate on ice with frequent stirring for 1 hour.
Add about 5 U DNAse, and MgCl2 to final concentration of 10mM. Stir at room temperature for 20-30 minutes.
Centrifuge at 20,000 rpm for 20 minutes. Collect supernatant and make 1mM PMSF. Store at -80°C until ready to perform purification procedure.
Apply filtered lysate (0.22mm) to Column 1 at about 0.8 mL/minute. Collect flow-through as a single fraction.
Wash Column 1 with 100 mL of buffer C. Collect wash as a single fraction.
Adjust the flow-through fraction from Column 1 to 5 mM CaCl2. Filter and apply to Column 2. Collect the flow-through as a single fraction.
Wash Column 2 as follows:Elute Column 2 with 100 mL of buffer C. Collect 7 mL fractions. The majority of Calmodulin will elute in the first 40 mL. A very small amount of pure protein may elute in the second A wash.
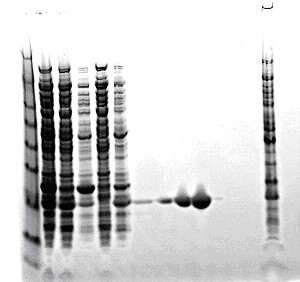
Analyze fractions by SDS-PAGE. Pool Calmodulin containing fractions and store at -80°C.
Note: the molar extinction coefficient of Calmodulin is 3006 M-1cm-1. By measuring the absorbance of the purified protein pool at 276 nm, an accurate determination of protein concentration can be obtained.