
Ca2+ Signal Transduction by EF-hand Proteins
EF-hand proteins play a role in most aspects of the Ca2+ signal transduction pathways, from regulation of Ca2+ channels to controlling the intensity and duration of Ca2+ signals to transduction of these signals into biochemical and biomechanical responses. The EF-hand is a very abundant protein motif, but despite its widespread distribution the underlying basis for Ca2+ signaling selectivity is not known. There is now a significant database of structural information about how EF-hand proteins respond to the binding of Ca2+ ions. This laid a foundation for the current emphasis on understanding propagation of signals downstream. Four primary areas of interest in studying EF-hand proteins are summarized below.
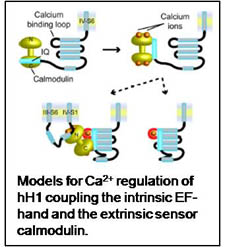
1. Ca2+ regulation of gating of the human cardiac Na+ channel (hH1).
Electrophysiological studies in the laboratory of my collaborator, Jeff Balser, demonstrated a Ca2+ effect on gating of hH1 that was originally assigned to Ca2+-dependent interactions between calmodulin and an IQ motif in the C-terminal region. However, subsequent analysis identified a functional EF-hand domain in the proximal C-terminal region of hH1. The structure of the EF-hand domain has been determined and mutations in this domain associated with long QT and Brugada syndromes were shown to be defective in Ca2+ binding (Shah 2006, Chagot 2009). In addition, we found that calmodulin can interact with the inactivation gate of the channel (Potet 2009). These data have been used to construct a model that couples the two Ca2+ sensing elements. Our ultimate goal is to fully elucidate the mechanism of Ca2+-dependent gating in hH1 and evaluate the potential of targeting this function for therapy in the treatment of cardiac arrhythmia syndromes.
2. Intracellular and extracellular functions of S100 proteins
S100s are a unique group of EF-hand proteins characterized by cell-type and cell cycle-specific expression, deregulated expression in certain diseases and the ability to be exported into the extracellular milieu. Once outside the cell, S100 proteins function through the activation of cell surface receptors and their unique ability to bind essential trace metals such as zinc, manganese and copper. A specific S100A8/A9 heterodimer termed calprotectin has been shown to be a critical element of innate immune system. We have shown in collaborative studies with the laboratory of Eric Skaar that calprotectin exerts a highly potent activity suppressing the growth of bacterial pathogens through starvation of essential trace metals (Corbin 2008). We are currently extending these studies by examining the trace metal specificity of this activity and the structural effects of metal binding.
Specific studies on S100A revelaed that is interacts physically and functionally with Siah-1 interacting protein (SIP), which serves to bridge Siah-1 and Skp1 in the SCFTBL1 complex. The association with SCFTBL1 is intriguing because it represents a new S100 protein function: involvement in regulation of protein ubiquitination. A specific role for S100A6 in localization of the SCFTBL1 complex is proposed because S100 proteins are known to interact with membranes in a Ca2+-dependent manner and S100A6 is known to translocate to membranes upon Ca2+ activation. In addition to the studies of SCFTBL1 described above, the structure of the complex of SIP and S100A6 has been determined and mutations were designed to test the function of the Ca2+-dependent interaction of S100A6 with SCFTBL1 in-vitro and in-vivo (Lee 2008).
3. Ca2+-dependent activation of the Receptor for Advanced Glycation Endproducts (RAGE)
RAGE is a cell surface receptor implicated in diabetic atherosclerosis and numerous inflammatory disorders. S100 proteins are secreted in the cell types in which RAGE exerts its activity, and they are known to modulate RAGE function. The S100A8/A9 heterodimer calprotectin (CP) is part of the innate immune system and a known RAGE ligand active in inflammatory settings. We collaborate with over 10 different laboratories on functional studies of CP and its interaction with RAGE, supplying recombinant protein and structural and biochemical expertise to interpret the data. Since the physical basis for RAGE signaling is not understood, our laboratory determined the structural organization of the three extracellular Ig-like domains of RAGE and its binding of S100 proteins (Dattilo 2007, Koch 2009). We are also using NMR spectroscopy and X-ray crystallography approaches to investigate the structural basis for recognition of S100 proteins and the mechanism of activation of RAGE.
4. The relationship between sequence, structure and function in EF-hand proteins
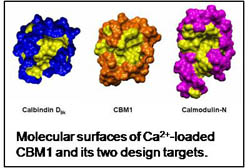
The large database of sequences and structures of EF-hand proteins provides a wealth of information that can be used to effectively correlate sequence and function. One of my goals is to learn how to effectively tap this goldmine to generate the ability to manipulate Ca2+ signaling proteins and use this capability for applications in therapeutics and biotechnology. Detailed comparative structural analyses of EF-hand proteins provide hypotheses about the critical residues or groups of residues in the sequence, which are tested and refined using an EF-hand protein engineering platform we term calbindomodulin (CBM) (Bunick 2004). The next phase of this research will involve coupled computational and experimental approaches designed to generate rational control of the conformational response of CBM to Ca2+ binding.
- Understanding the mechanics of multi-protein DNA processing machinery
- Structural, Biochemical, and Functional Studies of E3 Ubiquitin Ligases
- Ca2+ Signal Trasnduction by EF-hand Proteins