Structural and Chemical Biology of Membrane Proteins and Related Diseases
|
While about 1/3 of all proteins are membrane proteins, less than 0.2% of solved high resolution protein structures represent membrane proteins because of difficulties in applying classical methods of structural determinations to proteins of this class. Thus, it can be argued that membrane proteins are the greatest remaining frontier of structural biology. This area is particularly important because of the critical medical relevance of membrane proteins. Over 60% of all drug molecules target membrane proteins. Moreover, hundreds of diseases involve the misassembly or misfolding of integral membrane proteins. The Sanders lab is devoted to characterizing the structures, folding and misfolding, and molecular mechanisms of membrane proteins using a variety of biochemical and biophysical methods (but especially NMR). We are a biological problem-oriented lab. However, some of the problems we wish to solve require in-house development of new methods and technologies such as bicelles. |

|
|
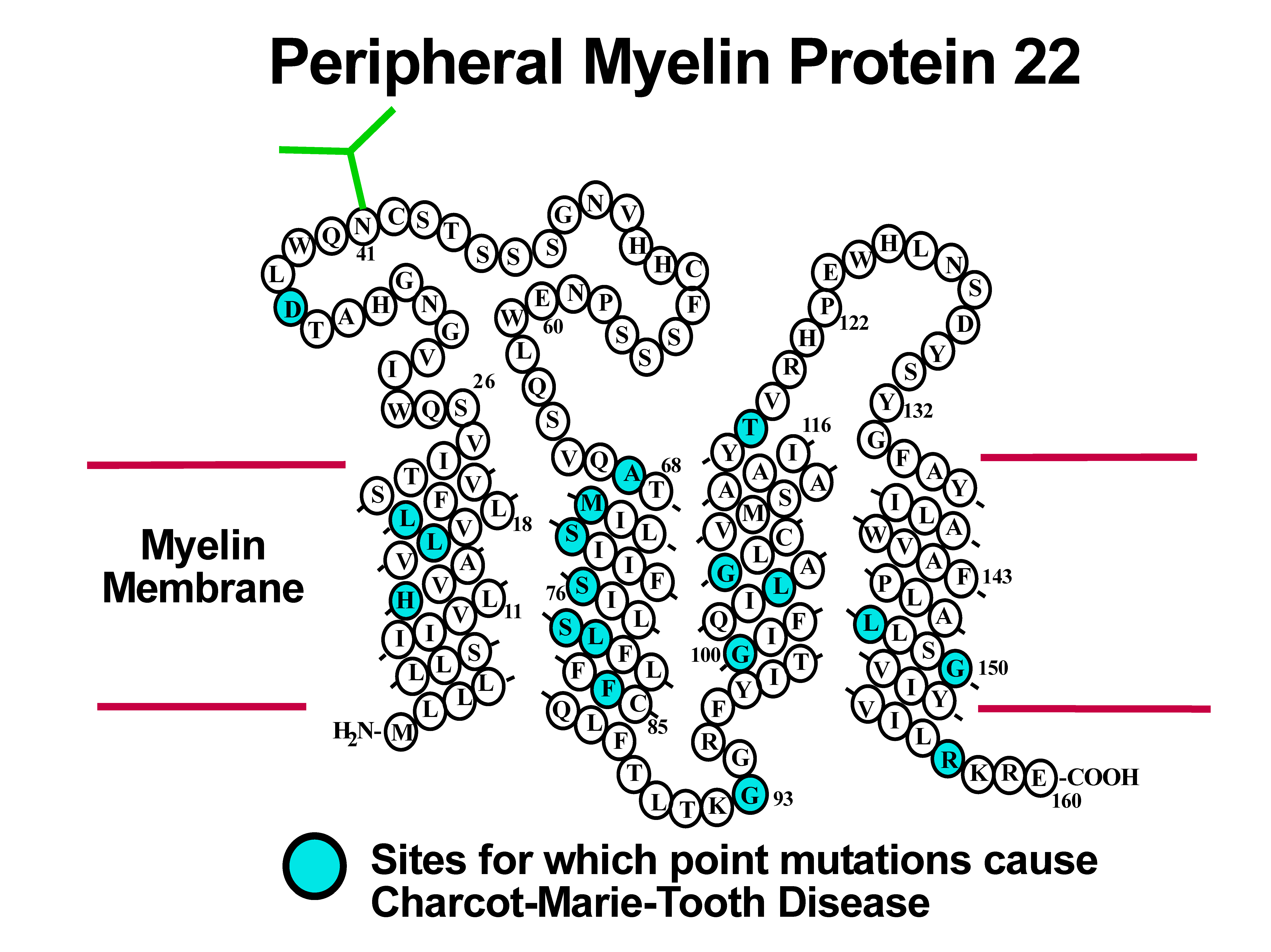
Many diseases are linked to protein misfolding induced by mutations or other factors.
Peripheral myelin protein 22 (PMP22) has four transmembrane segments and is a critical
component of the myelin sheath surrounding the axons of the peripheral nervous system.
Mutations in PMP22 that result in misfolding lead to Charcot-Marie-Tooth Disease, the most
common inherited disorder of the peripheral nervous system. Studies of the structures,
folding, stability, and molecular interactions of both wild type and disease-associated
mutants of human PMP22 are being conducted in order to illuminate how mutations in this
protein trigger misfolding and disease. |
|
 |
We are examining the regulation of the two human potassium channels that are involved in triggering heartbeat: HERG and KCNQ1. Mutations in these channels and the proteins that regulate them lead to heart disease. The Sanders lab is anchoring the structural end of collaborative studies of these proteins with the labs of Carlos Vanoye, Al George, Sabina Kupershmidt, Jeff Balser, and Jens Meiler. We seek to understand how proteins such as KCNE1 are able to bind to and profoundly alter the channel properties of KCNQ1 and HERG. |

|

|
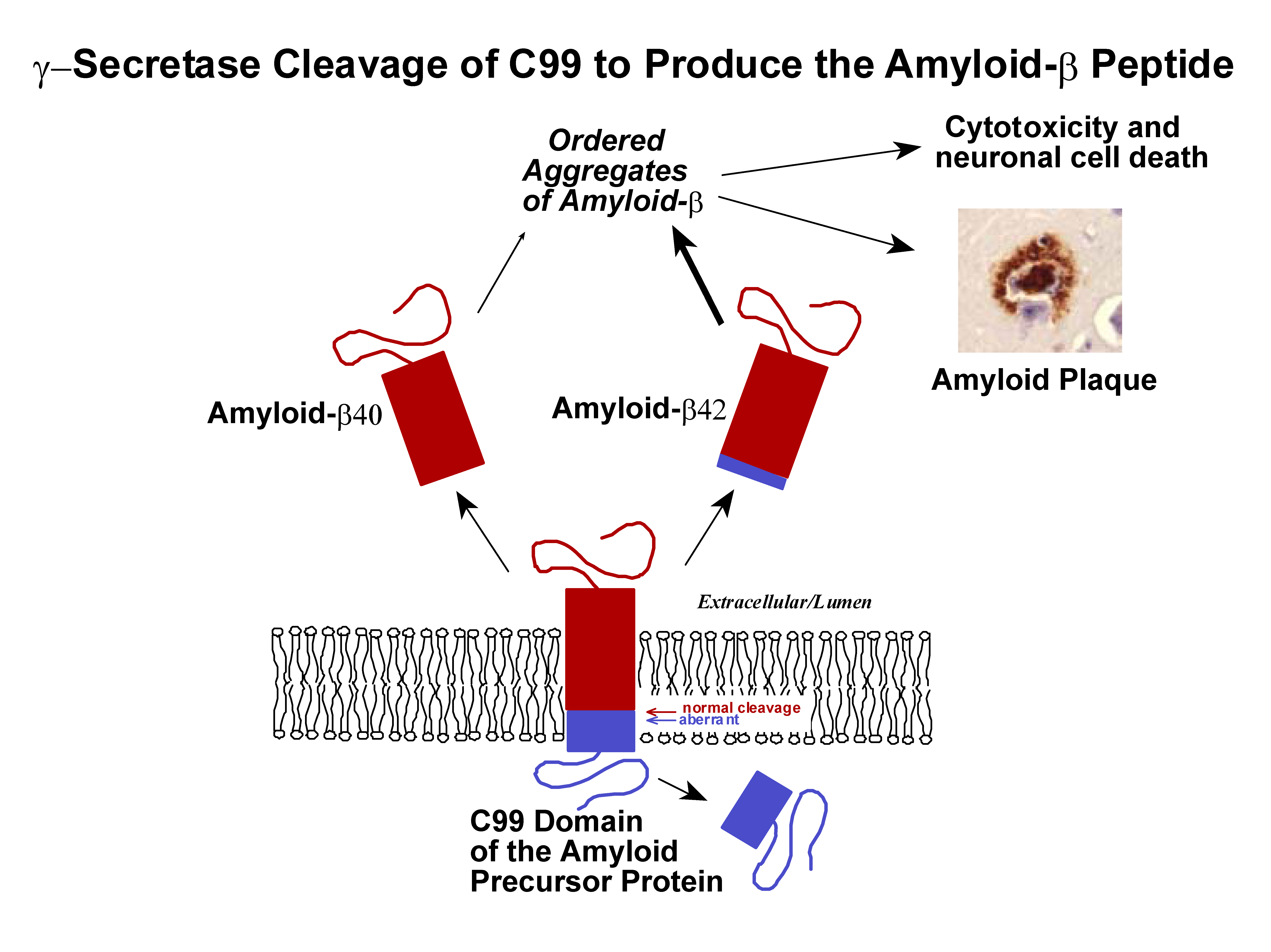
The amyloid-beta polypeptides that are believed to trigger Alzheimer’s disease are derived from the transmembrane amyloid precursor protein (APP). While much is known about the structural properties of the amyloid-beta polypeptides and the aggregates they form, much less is known about the structural biology of the competing pathways of APP cleavage that lead either to amyloid-beta formation or to non-amyloidogenic fragments. We are studying the structure of the critical transmembrane C99 domain of APP and its interactions with a variety of molecules that are believed to be involved in modulating its cleavage, including cholesterol. |
|
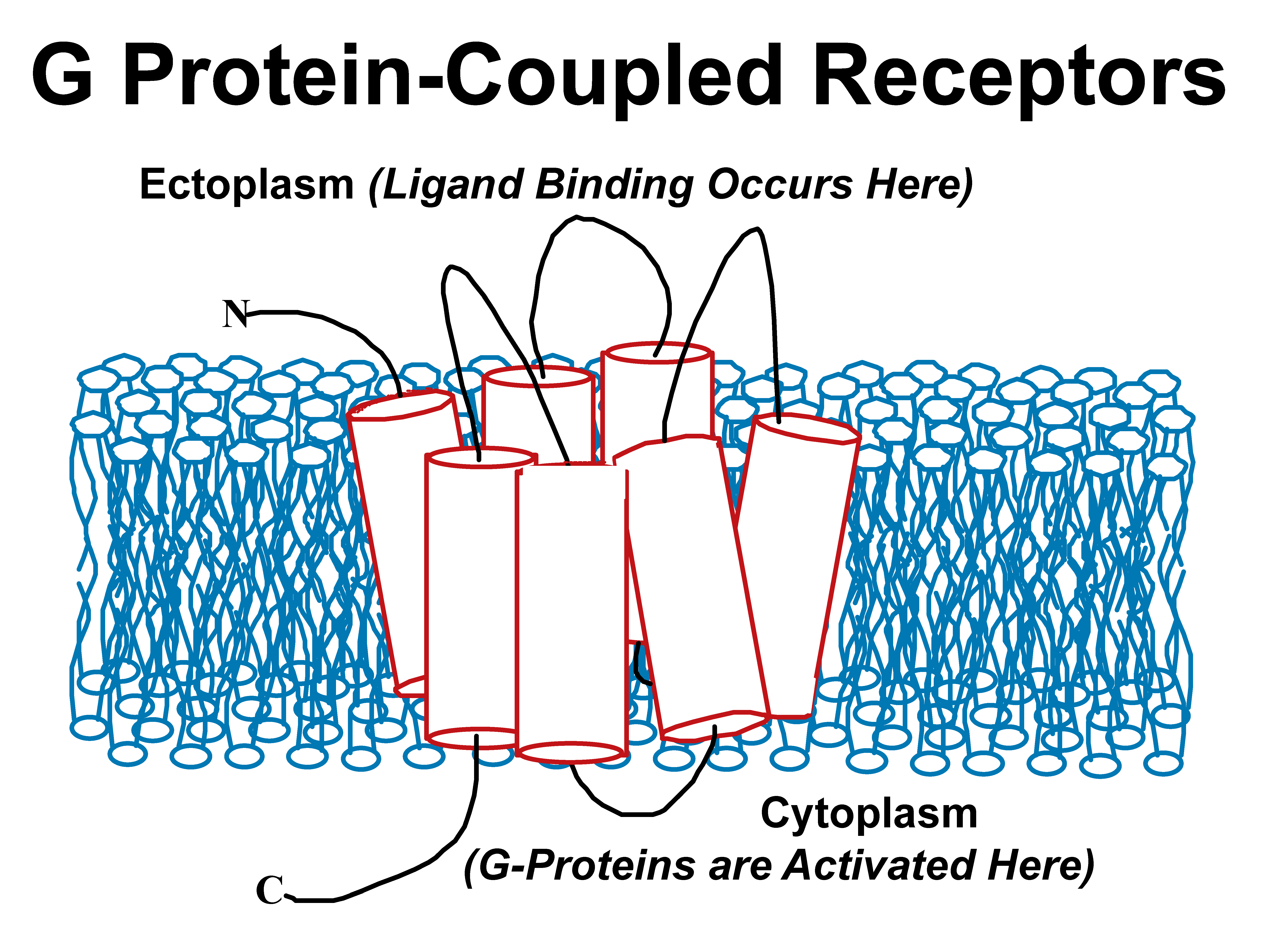 |
The human G protein-coupled receptor (GPCR) family has roughly 700 members and is the target of a sizable fraction of all current drugs. While there have been some recent and spectacular breakthroughs in X-ray crystallography of specific GPCRs, their structural biology remains at a early stage of development. In partnership with Profs. Rich Breyer (receptor pharmacology) and Tina Iverson (X-ray crystallography) we are pursuing structural studies of several human GPCRs directly involved in health and disease, including the vasopressin V2 receptor, the prostaglandin E2 receptor, and the kappa-opioid receptor. A particular focus of the Sanders lab is to bring solution NMR methods to bear on these proteins. |
|
|
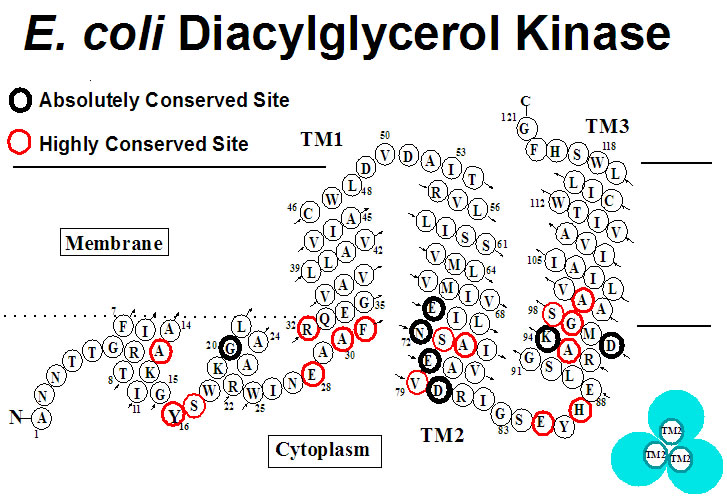
E. coli DAGK has three transmembrane segments per monomer and functions as a 40 kDa homotrimer. The prokaryotic form of this enzyme is structurally and functionally unrelated to eukaryotic DAGK and plays critical roles in microbial physiology. DAGK is also an important model system for understanding disease-related membrane protein folding and misfolding. We are studying DAGK’s structure, catalytic mechanism, how it folds and misfolds, and how it can be inhibited. |
|