Sanders Lab:
How Do Defects In Membrane Proteins Cause Disease?
|
Membrane proteins respresent a large and important class of membrane proteins that remain especially difficult to study. Over 60% of all drug molecules target membrane proteins. The theme of our lab's work is to elucidate how defects in membrane proteins contribute to human diseases. The Sanders lab is devoted to characterizing the structures, folding and misfolding, and molecular mechanisms of membrane proteins using a variety of biochemical and biophysical methods (including NMR). We are a biological problem-oriented lab. However, solving some of the problems we are interested in requires in-house development of new methods and technologies such as bicelles and other model membranes. |
 |
|
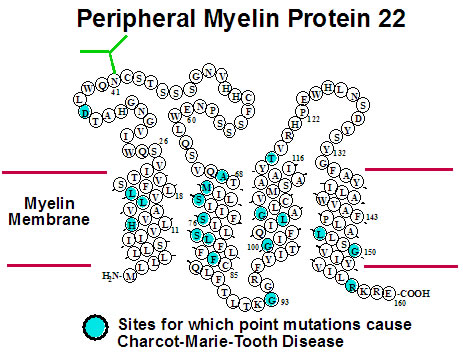
Many diseases are linked to protein misfolding induced by mutations or other factors.
Peripheral myelin protein 22 (PMP22) has four transmembrane segments and is a critical
component of the myelin sheath surrounding the axons of the peripheral nervous system.
Mutations in PMP22 that result in misfolding lead to Charcot-Marie-Tooth Disease, the most
common inherited disorder of the peripheral nervous system. Studies of the structures,
folding, stability, and molecular interactions of both wild type and disease-associated
mutants of human PMP22 are being conducted in order to illuminate how mutations in this
protein trigger misfolding and disease. We are also pursuing early-stage drug discovery for compounds that have the potential to treat CMTD by modulating its folding and trafficking in the cell. Collaborators on this project include Profs. Melanie Ohi (U Mich), Bruce Carter (VU), Anne Kenworthy (U Virginia), Jun Li (Wayne State), Carlos Vanoye (Northwestern U), Lars Plate, and Kevin Schey (VU). |
 |
|
We are examining the regulation of a human potassium channel involved in triggering heartbeat: KCNQ1. Mutations in this channels and the proteins that regulate it lead to heart disease, specifically long QT syndrome arrhythmia (LQTS). The Sanders lab is anchoring the structural end of collaborative studies of these proteins with the labs of Jens Meiler and Jarrod Smith (Vanderbilt), Carlos Vanoye and Al George (Northwestern), Jianmin Cui (Washington U) and Gary Lorigan (Miami U). We seek to understand how proteins such as KCNE1 are able to bind to and profoundly alter the channel properties of KCNQ1. We are also conducting early stage drug discovery for compunds that have the potential to treat LQTS by altering the folding and trafficking of the channel and/or its channel properties. |
 |
| The amyloid-beta polypeptides that are believed to trigger Alzheimer's disease are derived from the transmembrane amyloid precursor protein (APP). While much is known about the structural properties of the amyloid-beta polypeptides and the aggregates they form, much less is known about the structural biology of the competing pathways of APP cleavage that lead either to amyloid-beta formation or to non-amyloidogenic fragments. We are studying the structure of the critical transmembrane C99 domain of APP, its dimerization, and its interactions with a variety of molecules that are believed to be involved in modulating its cleavage, including cholesterol and GSAP (the gamma-secretase activating protein). Collaborators on this project are Profs. Anne Kenworthy (U Virginia) and Daniel Huster (U Leipzig, Germany), . |